10% (w/v) solution of Phe was prepared by dissolving dried Phe in THF with continuous stirring at 50 oC. Polymer nanocomposite (PN) films were made by mixing different loading percentage of phosphomolybdic acid modified red mud (PRM) and organically modified red mud (ORM) into the virgin Phe solution, stirred constantly and sonicated for half an hour for better dispersion. The composition of the PN materials was varied from 0 to 3wt % of modified RM with respect to the Phe content. The samples were designated as shown in Table 12.
|
Sample index |
% Loading of filler |
|
*Phe |
0 |
|
**PRC1 |
1.0 |
|
PRC2 |
2.0 |
|
PRC3 |
3.0 |
|
***PNC1 |
1.0 |
|
PNC2 |
2.0 |
|
PNC3 |
3.0 |
|
*Phe: Phenoxy or poly (hydroxy ether) of Bisphenol A ** PRC: Phe-phosphomolybdic acid modified red mud nanocomposites *** PNC: Phe-organically modified red mud nanocomposites |
Table 12. Sample Designation
1.5 FTIR spectroscopy
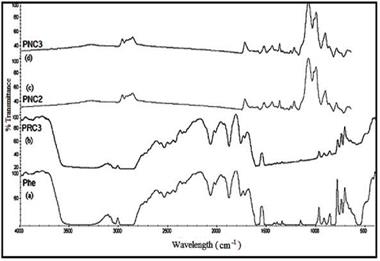
The FTIR spectra in Figure 23 showed the presence of characteristic peaks of poly (hydroxy ether) of bisphenol A. The characteristic vibration band of Phe for hydroxyl stretching vibration (O-H) having polymeric association was observed at 3394 cm-1, While the alkyl stretching band of C-H was shown at 2974 cm-1 and aryl stretching band of C-H at 3037 cm-1. The peak at v = 1183 cm-1 is due to C-O stretching of phenyl ether linkage. Further, the peak at v = 1607 cm-1 could be attributed to the aromatic ring.
Figure 23 also showed the spectra of Phe-modified red mud nanocomposite membranes with different loading percentage of the filler. The characteristic vibration band of PRC3 for hydroxyl stretching vibration (O-H) was observed at 3270 cm-1. The intensity of the O-H stretching vibration peak decreased and peak frequency shifted from 3394 to 3270 cm-1, i. e. to a lower frequency as compared to that of pure phenoxy. The red shift in the hydroxyl stretching frequency occurs due to the various degree of hydrogen bonding between the polymer and the filler which lengthens and weakens the O-H bond and hence lowers the vibrational frequency. The alkyl stretching band of C-H in case of PRC3 also showed red shift from 2974 to 2861 cm-1 due to the interaction of modified red mud with the polymer matrix. Similarly, peak at v = 3036 cm-1, was attributed to the stretching vibration of aryl C – H band, slightly lower frequency than the pure phenoxy. The other characteristic vibrational frequencies of PRC3 are presented in table 13.
|
Fig. 23. FTIR Spectrum of (a) Pure Phe (b) PRC3 (c) PNC2 (d) PNC3 |
|
Groups |
PHE |
PRC3 |
PNC2 |
PNC3 |
|
(cm-1) |
(cm-1) |
(cm-1) |
(cm-1) |
|
|
OH |
3394 |
3270 |
– |
– |
|
C-H |
2974 |
2861 |
2948 |
2947 |
|
C-H (Ar) |
3037 |
3036 |
3016 |
3020 |
|
NH3+ |
– |
– |
1571 |
1572 |
|
C-O |
1183 |
1168 |
1182 |
1180 |
|
C=O |
– |
1766 |
1760 |
1760 |
|
Ar |
1607 |
1613 |
1499 |
1503 |
|
Si-O |
– |
953 |
952 |
951 |
|
Mo-Oc-Mo |
– |
896 |
– |
– |
|
Table 13. Assignment of FTIR spectral bands for Pure Phenoxy and the Ph/modified red mud nanocomposite membranes |
The spectra of PNC2 and PNC3 as shown in fig. 23, showed the disappearance of the hydroxyl stretching frequency (O-H). The disappearance of the hydroxyl peak depicted complete physical interaction of the O-H group with different groups of the organically modified red mud, thereby not leaving any free hydroxyl group. However, alkyl stretching band of C-H for PNC2 and PNC3 were observed at 2948 and 2947 cm-1 respectively. The red shift in the alkyl stretching frequency C-H of PNC2 and PNC3 as compared to the pure phenoxy showed interaction of the filler with the polymer matrix.
The characteristic vibrational frequencies of PNC2 and PNC3 at v = 1571 and 1572 cm-1 respectively, was attributed to the stretching frequency of ammonium ion (NH3+), thereby depicting formation of zwitter ion. The other vibrational frequencies associated with PNC2 and PNC3 have been tabulated in table 13. The organically modified red mud(ORM) showed better interaction with the poly (hydroxy ether) of bisphenol A matrix as compared to phosphomolybdic acid modified red mud (PRM), therefore, it can be inferred that ORM will show better dispersion into the polymer matrix as compared to PRM.