Materials have enabled the advance of mankind from its earliest beginnings; indeed, the ages of mankind are named after the dominant material of the day: the Stone Age, the Age of Copper, the Bronze Age, the Iron Age (see Figure 1.1). The tools and weapons of prehistory, 300,000 or more years ago, were bone and stone. Stones could be shaped into tools, particularly flint and quartz, which could be flaked to produce a cutting edge that was harder, sharper, and more durable than any other material that could be found in nature. Simple but remarkably durable structures could be built from the materials of nature: stone and mud bricks for walls, wood for beams, rush and animal skins for weather protection.
Gold, silver, and copper, the only metals that occur in native form, must have been known from the earliest time, but the realization that they were ductile, could be beaten to complex shape, and, once beaten, become hard, seems to have occurred around 5500 B. C. There is evidence that by 4000 B. C. man had developed technology to melt and cast these metals, allowing more intricate shapes. Native copper, however, is not abundant. Copper occurs in far greater quantities as the minerals azurite and malachite. By 3500 B. C., kiln furnaces, developed to create pottery, could reach the temperature and create the atmosphere needed to reduce these minerals, enabling the development of tools, weapons, and ornaments that we associate with the Copper Age.
But even in a worked state, copper is not all that hard. Poor hardness means poor wear resistance; copper weapons and tools were easily blunted.
 |
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
 |
Sometime around 3000 B. C. the probably accidental inclusion of a tin-based mineral, cassiterite, in the copper ores provided the next step in technology: the production of the alloy bronze, a mixture of tin and copper. Tin gives bronze a hardness that pure copper cannot match, allowing the production of superior tools and weapons. This discovery of alloying—the hardening of one metal by adding another—stimulated such significant technological advances that it, too, became the name of an era: the Bronze Age.
Obsolescence sounds like 20th-century vocabulary, but the phenomenon is as old as technology itself. The discovery, around 1450 B. C., of ways to reduce ferrous oxides to make iron, a material with greater stiffness, strength, and hardness than any other then available, rendered bronze obsolete. Metallic iron was not entirely new: tiny quantities existed as the cores of meteors that had impacted the Earth. The oxides of iron, by contrast, are widely available, particularly hematite, Fe2O3. Hematite is easily reduced by carbon, although it takes high temperatures, close to 1100°C, to do it. This temperature is insufficient to melt iron, so the material produced was a spongy mass of solid iron intermixed with slag; this was reheated and hammered to expel the slag, then forged to the desired shape.
Iron revolutionized warfare and agriculture; indeed, it was so desirable that at one time it was worth more than gold. The casting of iron, however, presented a more difficult challenge, requiring temperatures around 1600°C. Two millennia passed before, in 1500 A. D., the blast furnace was developed, enabling the widespread use of cast iron. Cast iron allowed structures of a new type: the great bridges, railway terminals, and civic buildings of the early 19th century are testimony to it. But it was steel, made possible in industrial quantities by the Bessemer process of 1856, that gave iron its dominant role in structural design that it still holds today. For the next 150 years metals dominated manufacture. The demands of the expanding aircraft industry in the 1950s, with the development of the gas turbine, shifted emphasis to the light alloys (those based on aluminum, magnesium, and titanium) and to materials that could withstand the extreme temperatures of the jet combustion chamber (superalloys—heavily alloyed iron and nickel-based materials). The range of application of metals expanded into other fields, particularly those of chemical, petroleum, and nuclear engineering.
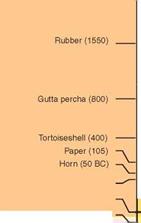
The history of polymers is rather different. Wood, of course, is a polymeric composite, one used for construction from the earliest times. The beauty of amber (petrified resin) and of horn and tortoise shell (the polymer keratin) already attracted designers as early as 80 B. C. and continued to do so into the 19th century. (There is still, in London, a Horners’ Guild, the trade association of those who worked horn and shell.) Rubber, brought to Europe in 1550, was already known and used in Mexico. Its use grew in importance in the 19th century, partly because of the wide spectrum of properties made possible by vulcanization—cross-linking by sulfur—giving us materials as elastic as latex and others as rigid as ebonite.
The real polymer revolution, however, had its beginnings in the early 20th century with the development of Bakelite, a phenolic, in 1909 and of synthetic butyl rubber in 1922. This was followed at midcentury by a period of rapid development of polymer science, visible as the dense group at the upper left of Figure 1.1. Almost all the polymers we use so widely today were developed in a 20-year span from 1940 to 1960, among them the bulk commodity polymers polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC), and polyurethane (PU), the combined annual tonnage of which now approaches that of steel. Designers seized on these new materials—cheap, brightly colored, and easily molded to complex shapes— to produce a spectrum of cheerfully ephemeral products. Design with polymers has since matured: they are now as important as metals in household products, automobile engineering, and, most recently, in aerospace.
The use of polymers in high-performance products requires a further step. "Pure" polymers do not have the stiffness and strength these applications demand; to provide those qualities they must be reinforced with ceramic or glass fillers and fibers, making composites. Composite technology is not new. Straw-reinforced mud brick (adobe) is one of the earliest of the materials of architecture, one still used today in parts of Africa and Asia. Steel-reinforced concrete—the material of shopping centers, road bridges, and apartment blocks—appeared just before 1850. Reinforcing concrete with steel gives it tensile strength where previously it had none, revolutionizing architectural design; it is now used in greater volume than any other manmade material. Reinforcing metals, already strong, took much longer, and even today metal matrix composites are few.
The period in which we now live might have been named the Polymer Age had it not coincided with yet another technical revolution, that based on silicon. Silicon was first identified as an element in 1823 but found few uses until the realization, in 1947, that, when doped with tiny levels of impurity, it could act as a rectifier. The discovery created the fields of electronics, mechatronics, and modern computer science, revolutionizing information storage, access and transmission, imaging, sensing and actuation, automation, real-time process control, and much more.
The 20th century saw other striking developments in materials technology. Superconduction, discovered in mercury and lead when cooled to 4.2°K (-269°C) in 1911, remained a scientific curiosity until, in the mid – 1980s, a complex oxide of barium, lanthanum, and copper was found to be superconducting at 30°K. This triggered a search for superconductors with yet higher transition temperatures, leading, in 1987, to one that worked
at the temperature of liquid nitrogen (98°K); making applications practical, though they remain few.
During the early 1990s it was realized that material behavior depended on scale and that the dependence was most evident when the scale was that of nanometers (10~9 m). Although the term nanoscience is new, technologies that use it are not. The ruby-red color of medieval stained glasses and the dia – chromic behavior of the decorative glaze known as "lustre" derive from gold nanoparticles trapped in the glass matrix. The light alloys of aerospace derive their strength from nanodispersions of intermetallic compounds. Automobile tires have, for years, been reinforced with nanoscale carbon. Modern nanotechnology gained prominence with the discovery that carbon could form stranger structures: spherical C60 molecules and rod-like tubes with diameters of a few nanometers. Now, with the advance of analytical tools capable of resolving and manipulating matter at the atomic level, the potential exists to build materials the way that nature does it, atom by atom and molecule by molecule.
If we now step back and view the timeline of Figure 1.1 as a whole, clusters of activity are apparent; there is one in Roman times, one around the end of the 18th century, one in the mid-20th. What was it that triggered the clusters? Scientific advances, certainly. The late 18th and early 19th centuries were a time of rapid development of inorganic chemistry, particularly electrochemistry, and it was this that allowed new elements to be isolated and identified. The mid-20th century saw the birth of polymer chemistry, spawning the polymers we use today and providing key concepts in unraveling the behavior of the materials of nature. But there may be more to it than that. Conflict stimulates science. The first of these two periods coincides with that of the Napoleonic Wars (1796-1815), one in which technology, particularly in France, developed rapidly. And the second was that of the Second World War (1939-1945), in which technology played a greater part than in any previous conflict. One hopes that scientific progress and advances in materials are possible without conflict, that the competitive drive of free markets is an equally strong driver of technology. It is interesting to reflect that more three quarters of all the materials scientists and engineers who have ever lived are alive today, and all of them are pursuing better materials and better ways to use them. Of one thing we can be certain: there are many more advances to come.



