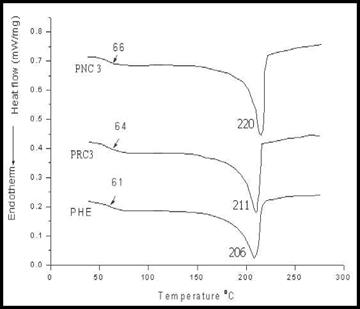
DSC traces of pure Phenoxy and polymer nanocomposite materials are shown in Fig.30. Ph exhibited a shallow endotherm at 61oC corresponding to the glass transition temperature (Tg) of Ph. All the nanocomposite materials with different critical loading percentage of modified red mud were found to have a high Tg as compared to the pristine Phe. This could be attributed to the confinement of the intercalated polymer chains within the red mud galleries that prevents the segmental motions of the polymer chains, thereby enhancing the glass transition temperature of the polymer matrix. The PNC3 nanocomposite membrane showed maximum Tg and melting temperature (Tm) of 66 oC and 220 oC respectively. This depicted that PNC3 is thermally more stable than PRC3 nanocomposite membrane on account of better dispersion and adhesion of the ORM within the polymer matrix. The glass transition temperature (Tg) and melting temperature (Tm) of pure phenoxy and Ph nanocomposite membranes have been tabulated in table 17.
|
Sample |
Tg (o C) |
H 3 0 n |
|
Pure Phe |
61 |
206 |
|
PNC3 |
64 |
211 |
|
PRC3 |
66 |
220 |
|
|
Fig. 30. DSC thermograms of (a) Ph (b) PRC3 (c) PNC3
1.6 Conclusion
– Red mud was successfully modified with the inorganic acids and organic moiety (aniline formaldehyde). The modification ameliorates both the dispersion level and the material properties of the polymer nanocomposites.
– The PVA – boric acid modified red mud nanocomposite films showed better glass transition temperature Tg and thermal stability at a filler concentration of 3.0wt percentage.
– In PVA/ORM nanocomposite systems, 2.0wt% modified red mud (CP4) based nanocomposite film exhibited relatively good dispersion with increase in the thermal stability, glass transition temperature.
– In PVA/PRM nanocomposite systems, 2.5wt% modified red mud (PRM4) based nanocomposite film exhibited relatively good dispersion with increase in the material properties as compared to the pristine poly (vinyl alcohol).
– PVA based nanocomposites showed an increase in the roughness values with the increase in the filler content but the 3.0wt% of boric acid modified red mud nanocomposite membrane showed lower roughness values as compared to the critical loading percentage of ORM and PRM based nanocomposites.
– On comparing the material properties of all the three nanocomposite systems, it was found that the critical loading percentage of BRM based nanocomposite system (i. e., SP5) showed better enhancement as compared to the critical loading percentage of ORM and PRM based nanocomposite systems.
– The TEM images of the PVA-modified red mud nanocomposite membranes showed homogeneous nano phase dispersion of the modified red mud in the PVA polymer matrices at a scale of 8- 23 nm particle size.
– In the poly(hydroxy ether of bisphenol A) (Phe) based nanocomposites as reported in this work, mostly intercalated structures were produced in the acid modified red mud modifications, but modification by organic moiety (ORM) resulted in a mixed intercalated-exfoliated structure.
– Thermal stability of polymer also increases after nanocomposites preparation, because red mud acts as a heat barrier, which enhances the overall thermal stability of the system, as well as assist in the formation of char after thermal decomposition.
– Phe based nanocomposites showed an increase in the roughness values with the increase in the filler content but the 3.0wt% of organically modified red mud nanocomposite membrane showed lower roughness values as compared to the critical loading percentage of PRM based nanocomposites. Thus, PNC3 showed homogenous and non-porous morphology as compared to the coarse morphology of PRC3 type of nanocomposites.
– The average particle size in case of PNC3 is 14 nm while the particle size of PRC3 is 19 nm as revealed by TEM studies.
5. References
Agag, T.; Koga, T.; Takeichi, T. (2001). Studies on thermal and mechanical properties of polyimide-clay nanocomposites, Polymer, 42, 3399.
Alexandre, M.; Dubois P. (2000). Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Materials Science & Engineering, R: Reports, R28 (1-2), 1-63.
Bachtsi, A. R; Kiparissides, C. (1996). Synthesis and release studies of oil containing poly (vinyl alcohol) microcapsules prepared by coacervation, Journal of Controlled Release, 38 (1), 49-58.
Bhat, A. H, Banthia, A. K, (2007). Preparation and Characterization of Poly (vinyl alcohol)- Modified red mud composite materials, Journal of Applied Polymer Science, 103 (1), pp. 238-243.
Biron, M., (2007). Thermoplastics and Thermoplastic Composites. Elsevier Ltd., Jordan Hill, Oxford.
Chand, N.; Hashmi, S. A. R. (1999). Effect of Red Mud Addition on Abrasive Wear Rate of Isotactic Polypropylene/Low Density Polyethylene Blend under Low Stress Conditions, J Sci Ind Res, 58, 795.
Chand, N.; Hashmi, S. A. R. (1999). Effect of addition of polycarbonate on sheared flow of red mud-filled isotactic polypropylene Bull Mater Sci, 22, 801.
Chen, H. L. Wu, L. G; Tan, J; Zhu, C. L.; (2000). PVA membrane filled P-cyclodextrin for separation of isomeric xylenes by pervaporation. Chemical Engineering Journal, 78 (23), 159-164.
Dilsiz, N.; Ebert, E.; Weisweiler, W.; Akovali, G. (1995). Effect of plasma Polymerization on Carbon Fibers Used forFiber/Epoxy Composites J Colloid Interface Sci, 170, 241.
Donnet, J. B.; Bansal, R. C. Carbon Fibers, 2nd ed.; Dekker: New York, 1990.
Fornes TD, Hunter DL, Paul DR. (2004) Nylon – 6 nanocomposites from alkylammonium – modified clay: the role of alkyl tails on exfoliation. Macromolecules, 37(5), pp. 17931798.
Hajji, P.; David, L.; Gerard, J. F; Pascault, J. P; Vigier, G. (1999). Synthesis, structure, and morphology of polymer-silica hybrid nanocomposites based on hydroxyethyl methacrylate, Journal of Polymer Science, Part B: Polymer Physics, 37(22), 3172-3187.
Horii, F; Hu, S; Ito, T; Odani, H; Kitamaru. R, (1992).Cross-polarization/magic-angle-spinning carbon-13 NMR study of solid structure and hydrogen bonding of poly(vinyl alcohol) films with different tacticities, Polymer, , 33(11), 2299-2306.
Huang, H H.; Orler, B.; Wilkes, G L. (1987). Structure-property behavior of new hybrid materials incorporating oligomeric species into sol-gel glasses. 3. Effect of acid content, tetraethoxysilane content, and molecular weight of poly (dimethylsiloxane), Macromolecules, 20, 1322.
Ishikawa, Y.; Matsumoto, Y. (2001). Electrodeposition of TiO2 photocatalyst into nano – pores of hard alumite Electrochem Acta, 46, 2819.
Jang, B. Z. (1992). Control of interfacial adhesion in continuous carbon and kevlar fiber reinforced polymer composites, Compos Sci Technol, 44, 333.
Kasliwal, P.; Sai, P. S. T. (1999). Enrichment of titanium dioxide in red mud: a kinetic study Hydrometallurgy, 53, 73.
Lee, D. C.; Jang, L. W. (1996). Preparation and characterization of PMMA-clay hybrid composite by emulsion polymerization. Journal of Applied Polymer Science, 61(7), pp. 1117-1122.
Novak, B. M; Ellsworth, M. W. (1993). "Inverse" organic-inorganic composite materials: high glass content non – shrinking sol-gel composites, Materials Science & Engineering, A: Structural Materials: Properties, Microstructure and Processing, A162(1-2), 257- 64.
Park, S. J.; Cho, M. S. (2000). Effect of anti-oxidative filler on the interfacial mechanical properties of carbon-carbon composites measured at high temperature. Carbon, 38, 1053.
Park, S. J.; Kim, M. H. (2000). Effect of acidic anode treatment on carbon fibers for increasing fiber – matrix adhesion and its relationship to interlaminar shear strength of composites J Mater Sci, 35, 1901.
Park, S. J.; Kim, J. S. (2001).Influence of Plasma Treatment on Microstructures and Acid – Base Surface Energetics of Nanostructured Carbon Blacks: N2 Plasma
Environment, J Colloid Interface Sci, 232, 311.
Peppas, N. A; Merrill, E. W, (1977). Development of semicrystalline poly(vinyl alcohol) hydrogels for biomedical applications, Journal of Biomedical Materials Research, 11(3), 423-34.
Pradhan, J.; Das, S. N.; Thakur, R. S. (1999). Adsorption of Hexavalent Chromium from Aqueous Solution by Usin Activated Red Mud, J Colloid Interface Sci, 217, 137.
Ray S. S and Bousmina M, (2005). Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world, Progress in Materials Science, 50(8), 962-1079.
Shao, C.; Kim, H-Y; Gong, J.; Ding, B.; Lee, D-R.; Park, S-J. (2003). Fiber mats of poly(vinyl alcohol)/silica composite via electrospinning, Materials Letters, 57(9-10),1579-1584.
Surivet, F.; Lam, T. M ; Pascault, J. P; Pham, Q. T. (1992). Organic-inorganic hybrid materials. 1. Hydrolysis and condensation mechanisms involved in alkoxysilane – terminated macromonomers, Macromolecules, 25(17), 4309-20.
Yuan, L. T.; Shyu, S. S.; Lai, J. Y. (1991). Plasma surface treatments on carbon filbers. II. Mechanical property and interfacial shear strength J Appl Polym Sci, 42, 2525.
Zhang H, Ma H, Hongtu Li and Wang (2002). J, Synthesis and characterization of a polymer – metal complex from bisphenol A polyhydroxy ether with sulfur-bearing side-groups, Polym Int., 51, pp.1129-1134.