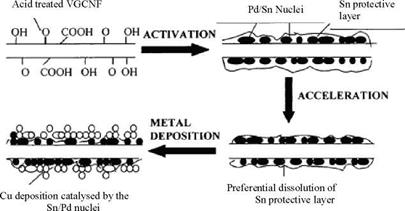
After acid and ultrasonic treatments, the VGCNF are subjected to 3 consecutive steps (fig. 5). The sensitization (Sn absorption), activation (Pd absorption), and plating (Cu deposition) stages consist in the successive immersion of the VGCNF into the following baths:
• Sensitization bath: 10g/l SnCl2, 40ml/l HCl 37%, distilled water;
• Activation bath: 0.25 g/l PdCl2, 2.5 ml/l HCl 37%, distilled water;
• Plating bath: 10g/l CuSC>4, 50g/l KNaC4H4O6-4H2O, 10 g/l NaOH, 15 ml/l CHOH distilled water
|
Materials |
Specific surface area (m2.g-1) |
Mean particle diameter (bm) |
Apparent density (g. cm-3) |
Density (g. cm-3) |
CTE (10-6 /°C) |
Thermal conductivity (W. m-1.K-1) |
Young’s modulus (GPa) |
|
Spherical copper powder (Nanoval) |
0.05-0.06 |
10-15 |
5.1 |
8.9 |
17 |
400 |
120 |
|
Electrolytic Copper powder (CH-UF 10) |
0.3 |
5-10 |
1.8 |
8.9 |
17 |
400 |
120 |
|
Picht carbon fibres (KT120) |
0.4 |
10 in diameter |
(<1) Not applicable |
2.12 |
-1,2 // |
1-5 1 140 // |
5-20 1 640 // |
|
PAN carbon fibre (T300) |
0.4 |
10 in diameter |
(<1) Not applicable |
2.10 |
-1,2 // |
1-5 1 70 // |
1-5 1 220 // |
|
Vapour growth carbon nano fibres |
13 |
0.06-0.15 in diameter |
(<1) Not applicable |
2.12 |
-1,2 // |
1200 // |
– |
|
Table 2. Main characteristics of copper powders and carbon fibres. |
|
Fig. 5. General principles of electroless coating [Lang et al., 1999] |
CHOH initiates the coating deposition by reducing the CuSO4 according to the following reaction:
Cu2+ + 2HCHO + 4OH – ^ Cu0 + H2 + 2H2O + 2HCOr.
desired pH), 2) the deposition time, 3) the temperature of the different baths and 4) the reaction rates.
After each immersion, the VGCNF are immediately rinsed with distilled water to stop the deposition process and, after the last step, the samples are carefully left dry.
Figures 6 and 7 show the evolution of the VGCNF Cu coating with different conditions. It has to be mentioned that the VGCNF have been treated with an acid solution (VGCNF + (HNO3 + H2SO4 2/3 vol.) during 6hours. before electroless coating.
Several point s can be noticed from this study:
1. The pH of the solution is one of the key factors in order to obtain a homogeneous copper coating around each VGCNF. Homogeneous coating is obtained for pH greater or equal to 12 (fig. 6). For that pH value the VGCNF are mostly cover with homogeneous coating and the coating thickness can easily be controlled with parameters as Cu sulfate concentration, deposition time and VGCNF content.
2. Copper coating is more homogeneous when i) the reducer concentration (HCHO) is increased and ii) the water content of the solution is increased (fig. 7). Both conditions lead to a decrease of the coating speed (see for example fig. 7a and 7b for two water content 100 ml and 200 ml). The diagrams linked with these 2 water contents (fig. 7d and 7f) show the evolution of the bath temperature and Ph with the deposition time. For each diagram, the diagram background color (gray to white) shows the evolution of the color of the solution (non transparent from Ph going to 13 to 10 and transparent when all the Cu salt has reacted and therefore for a pH lower than 10) with reaction time. Whatever the starting point and solution the advancement of the reaction is linked with a decrease of the Ph and an increase of the solution temperature (the exothermicity of the reaction is linked with the VGCNF concentration within the bath)
3. Whatever the starting conditions, an increase of the bath temperature is always observed and linked with the decrease of the pH of the solution.