Organic fertilizers tend to be low in nutrient content, especially nitrogen. Although they enjoy some popularity with organic gardeners and hobbyists, they have limited application to commercial ornamental horticulture. Not only are the nutrients limited in the organics, but they are often more slowly available to the plant than those in chemical fertilizers. Organic fertilizers include materials such as dried blood, cocoa meal, animal manures, dried sewage sludge, and bone meal. The latter two materials have some commercial usage. Sewage sludge is used as a top-dressing on golf greens and bone meal as a high-phosphorus fertilizer for flower bulbs.
|
py TABLE 3-4. |
Comparison of High- |
and Low-Analysis Fertilizers |
|
High-Analysis Fertilizers |
Low-Analysis Fertilizers |
|
|
Contain more nutrients and less filler |
Contain fewer nutrients and more filler |
|
|
Cost less per pound of actual nutrient |
Cost more per pound of actual nutrient |
|
|
Weigh less; less labor required in handling |
Weigh more and are bulkier; more labor required in handling |
|
|
Require less storage space |
Require more storage space |
|
|
Require less material to provide a given amount of nutrients per square foot |
Require more material to provide a given amount of nutrients per square foot |
|
|
Require less time to apply a given amount of nutrients Require more time to apply a given amount of nutrients |
Ammonification and Nitrification
The presence of an element in the soil does not guarantee that the plant can make use of it. It may be an essential element but in a form that must undergo chemical change before being available to the plant. Nitrogen is made available in green plants as nitrate salts (NO-3) or ammonium salts (NH+4). In turn, members of the animal kingdom depend almost exclusively on green plants for their nitrogen that they take from plants’ proteins and amino acids.
Nitrogen salts are not found as minerals in the soil. They are formed by the decomposition of nonliving plants, plant parts, animals, or excretory products. Integral to this degradation are several separate groups of bacteria that are key factors in the twin conversions known as ammonification and nitrification.
Ammonification is the conversion of nitrogen in organic compounds to ammonia. Nitrification is the conversion of ammonia to nitrite, then to nitrate as shown.
2 NH+4 + 4O2 ^ 2 NO-2 + 4H2O + energy (ammonia) (nitrite)
2 NO-2 + O2 ^ 2 NO-3 + energy (nitrite) (nitrate)
The energy produced by these reactions is used by the bacteria for their growth and development.
Collectively, the two processes can be diagrammed as follows:
nitrite nitrate
organic nitrogen ^ ammonium salts (NH+4) ^ salts (NO-2) ^ salts (NO-3) Ammonification Nitrification
Three additional points deserve mention. First, the nitrifying bacteria are sensitive to low temperatures, acidity, and poor drainage. In such conditions, the bacteria do not drive the biochemical changes as rapidly as they do under more favorable conditions. Second, the microorganisms of the soil need nitrogen for growth as much as green plants or animals do. As a result, the addition of large quantities of organic matter to the soil can result in a temporary decline in the amount of nitrogen available to crop plants as the soil microbial population increases. Finally, three genera of bacteria (Azotobacter, Clostridium, and Rhizobium) are able to convert atmospheric nitrogen gas (N2) into organic compounds. After the bacteria die, the processes of ammoni – fication and nitrification release the nitrogen for use by green plants. Especially important is the relationship between the Rhizobium bacteria and members of the bean family (the legumes or Leguminosae). The bacteria live in the legume roots where they obtain organic food materials. In return, the bacteria capture or fix nitrogen gas, making it available to the green plant.
Phosphorus
Phosphorus is present in very small amounts in mineral soils. An even smaller amount is present in a form usable by plants at any one time, since even simple phosphorus compounds are usually insoluble in the soil solution. Along with nitrogen, which is also present in small amounts, phosphorus is often a limiting factor in soil nutrition.
Much of the soil’s phosphorus is bound within the organic matter of the soil and becomes available to plants as organic material decomposes. The phosphorus held within the mineral matter of the soil is much more slowly available to the plant. Only through a slow interaction between the insoluble phosphate, water, carbon dioxide, and various root exudates does the phosphate become water-soluble, and, hence, available. Even then, the soluble phosphates can quickly revert to complex, insoluble forms, especially as the pH increases or decreases.
Potassium
Potassium is present in soil in much larger quantities than either nitrogen or phosphorus. Nearly all the soil’s potassium is in inorganic forms, however, and inorganic potassium is not readily available to plants. It becomes available slowly through the reaction of water and carbonic acid in the soil with the feldspars, micas, and other sources of insoluble potassium.
The other essential elements require similar chemical reaction in the soil to become available for absorption by the plant. Soil chemistry is a complex field of study and the brief treatment given the subject here has only touched on a few of the points necessary to an understanding of crop production.
SUMMARY______________________________________
Soil is the thin outer layer of the earth’s crust, made up of weathered minerals, living and nonliving organisms, water, and air. It provides the underground environment of plants. In profile, three distinctive layers can be seen: bedrock or parent material, subsoil, and topsoil. Both subsoil and topsoil are finely weathered from the parent material, but topsoil contains more organic matter than subsoil. As a result, it is more supportive of plant growth.
Organic matter is composed of plant and animal compounds that soil microbes can rapidly break down, and of humus. Humus is a complex colloidal mixture that originates with organic compounds that do not decompose easily. Humus and other organic matter are important in the development of good soil structure, causing small soil particles to bind and form larger particles or aggregates.
Soils differ in many ways, such as color, weight, drainage, rockiness, and texture. The differences result from weathering elements, soil movement, topography, climate, and variations in the amount of organic matter.
When parent material weathers, particles of differing sizes are formed. The four groups of weathered particles or separates are gravel, sand, silt, and clay. The relative proportion of these separates in any one soil creates the soil texture. The textural name of a soil roughly describes the particular mixture of separates that it contains.
Because of their colloidal properties, clay and humus are instrumental in making nutrients available to plants. Seventeen nutrients have been found essential to the growth of green plants (C, H, O, P, K, N, S, Ca, Mg, Fe, Mo, B, Cu, Mn, Zn, CI, Ni). Of these essential elements, only carbon, hydrogen, and oxygen are obtained by the plant from sources other than the soil. The remainder are absorbed as minerals from the soil around plant roots. When one or more elements is lacking totally
or in part, is bound in a form unavailable to the plant, or is part of an overall nutrient imbalance in the soil, the plant will exhibit symptoms of nutrient deficiency.
Several other elements have been found to be beneficial to plant growth, even though they are not essential to completion of the plant life cycle.
The soil solution is a liquid composed of water held within the soil and mineral salts dissolved in the water. How the soil solution reacts chemically determines its acidity or alkalinity and is expressed as a pH number. Soils that contain more hydrogen ions (H+) than hydroxyl ions (OH-) are acidic and have a pH of less than 7.0. Soils containing more hydroxyl ions than hydrogen ions are alkaline and have a pH greater than 7.0. A soil with equal concentrations of hydrogen and hydroxyl ions is neutral and has a pH of 7.0. Soil additives can raise or lower the pH of the soil depending on whether they increase the number of H+ ions or OH – ions.
Modification of pH and nutrient uptake by plants depend on cation exchange: the capacity of colloidal particles to attract positively charged ions (cations) and exchange one ion for another. Those soils with a higher percentage of colloidal particles, such as clays and organic soils, have the highest cation exchange capacity. If soil pH becomes too high or too low, certain elements become bound tightly within the soil and unavailable to the plants.
Fertilizers are nutrient additives applied to the soil periodically to maintain optimum crop productivity. They may be complete or incomplete, high-analysis or low-analysis, inorganic (chemical) or organic. Usually, the elements within the fertilizer must undergo chemical changes before becoming available to the plant. Ammonification and nitrification exemplify such changes.
 |
 |
 |
|
ACHIEVEMENT REVIEW
|
Fertilizer Characteristic |
Low-Analysis Fertilizer |
High-Analysis Fertilizer |
All Complete Fertilizers |
|
Contains N, P, and K |
|||
|
Less than 30 percent of its weight is in available nutrients |
|||
|
Most bulky to store and handle |
|||
|
Contains 30 percent or more of its weight in available nutrients |
|||
|
Costs more per pound of nutrients |
|||
|
Requires less time to apply a given amount of nutrient |
|||
|
Contains filler material |
|||
|
Requires less material to apply a given amount of nutrients per square foot |
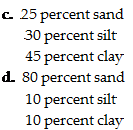
 |
 |



