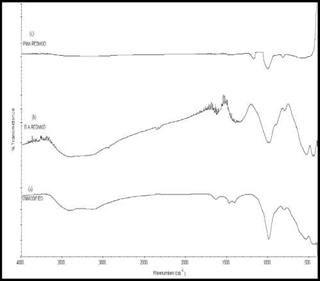
The nature of the chemical bonds in raw red mud and the modified red mud were characterized by FTIR spectroscopy. The FTIR spectra of raw red mud and acid modified red mud are shown in Fig. 5 and it clearly depicts the main characteristic peaks associated with them. The characteristic vibration band of raw red mud for hydroxyl stretching vibration (O-H) was found at 3417 cm-1 while for boric acid modified red mud, the hydroxyl stretching occurred at 3379 cm-1 and the same characteristic peak for phosphomolybdic acid was observed at 3129 cm-1. A red shift in the hydroxyl stretching vibration (O-H) at 3379 cm – 1 in the boric acid modified red mud (BRM) and 3129 cm-1 in the phosphomolybdic acid modified red mud (PRM) arises due to disappearance of the hydroxyl groups upon cross linking reaction with the boric acid and the phosphomolybdic acid respectively. The peak at и = 993 cm-1 in case of raw red mud could be attributed to Si-O stretching vibration and the same peak for BRM and PRM were observed at 1000 cm-1 and 996 cm-1 respectively. The blue shift observed in the peak position of Si-O occurred due to the interaction of red mud with the inorganic acids.
|
Fig. 5. FTIR Spectra of (a) Raw red mud (b) Boric acid modified red mud (BRM) (c) Phosphomolybdic acid modified red mud (PRM) |
According to the Rocchiccioli – Deltcheff et al. [80], the four absorption bands in the spectrum of pure phosphomolybdic acid (PMA) shows the typical features of Keggin anions and the peak frequencies at 1064, 961, 868, 780 cm-1 are assigned to then v (P-O),n v (MoOt) (Ot refers to the terminal oxygen), □ v (Mo-Oc-Mo) (Oc refers to the corner oxygen) andn v (Mo-Oe-Mo) (Oe refers to the edge oxygen) respectively. All these characteristic bands of PMA are not present in the spectra of the PRM, indicating that there is specific interaction between the keggin structure and red mud.
|
Groups |
Raw red mud (cm-1) |
BRM (cm-1) |
PRM (cm-1) |
|
OH |
3417 |
3379 |
3129 |
|
OH bend. |
1636 |
1631 |
– |
|
B-O |
– |
1368 |
– |
|
Si-O |
993 |
1000 |
996 |
|
P-O |
– |
– |
1074 |
|
Mo-Oc-Mo |
872 |
|
Table 3. Assignment of FTIR spectral bands for raw red mud and the acid modified red mud |
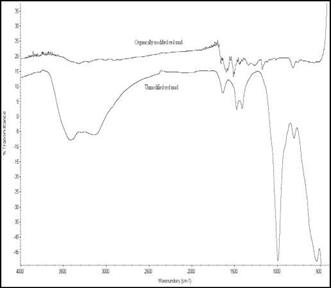
An upward shift was observed for the v (P-O) and v (Mo-Oc-Mo) stretching vibrations, whereas bands ascribed to the stretching vibration v (Mo-Ot) does not appear in the PRM. The major vibration bands for organically modified red mud as shown in Fig. 6 occurs at OH/NH stretching (3320 cm-1 ), NH3+ (1598 cm-1) and Ar-N (1512 cm-1). A red shift in the hydroxyl peak at 3320cm-1 for modified red mud arises due to the interaction of red mud platelets with the aniline hydrochloride moiety through physical interactions. The characteristic Si-O stretching vibration is not found in case of ORM due to formation of a coating of organic moiety around the silicate galleries.
|
Fig. 6. FTIR Spectra of Raw red mud and organically modified red mud (ORM) |
|
Groups |
Raw red mud |
ORM |
|
(cm-1) |
(cm-1) |
|
|
OH |
3417 |
3320 |
|
OH (bending) |
1636 |
– |
|
NH3+ |
– |
1598 |
|
Ar-N |
– |
1512 |
|
Si-O |
993 |
– |
|
Table 4. Assignment of FTIR spectral bands for raw red mud and organically modified red mud |
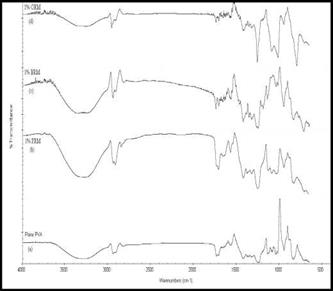
The representative FTIR spectra of the pure PVA and various polymer nanocomposite materials are shown in Fig.7.The characteristic vibration band of PVA for hydroxyl stretching vibration(O-H) having polymeric association is shown at 3292 cm-1, While the alkyl stretching band of C-H is shown at 2942 cm-1.The peak at n=1736 cm-1 is due to C=O stretching of saturated aliphatic esters (acetate), 1042cm-1(O-H Bending), and 1424 cm-1 (C-H Bending).
The FTIR spectrum in Fig. 3.6 also shows the spectra associated with PRM2, SP2 and CP2. The intensity of the O-H stretching vibration peak (v = 3200-3400 cm-1) decreased and peak frequency shifted from 3292 to 3250 cm-1, i. e. to a lower frequency as compared to that of pure PVA. The red shift in the hydroxyl stretching frequency occurs due to the various degree of hydrogen bonding between the polymer and the filler which lengthens and weakens the O-H bond and hence lowers the vibrational frequency. Also, the peak frequency of C-H for PRM2, SP2 and CP2 occurs at 2916 cm-1, 2945 cm-1 and 2960 cm-1 respectively as tabulated in Table 5.
By loading 1% ORM (CP2) in the polymer matrix, the alkyl stretching vibration of C-H increases from 2942-2960 cm-1 and occurs due to the interaction of polymer matrix with the organic moiety of the filler.
|
Fig. 7. FTIR Spectra of (a) Pure PVA (b) PRM2 (c) SP2 (d) CP2 |
|
Groups |
PVA (cm-1) |
PRM2 (cm-1) |
SP2 (cm-1) |
CP2 (cm-1) |
|
OH |
3292 |
3268 |
3267 |
3250 |
|
C-H |
2942 |
2916 |
2945 |
2960 |
|
C=O |
1736 |
1712 |
1743 |
1742 |
|
O-H (bending) |
1042 |
1043 |
1041 |
1019 |
|
CH2 (bending) |
1424 |
1423 |
1424 |
1414 |
|
Table 5. Assignment of FTIR spectral bands for Pure PVA and the PVA/modified red mud nanocomposite membranes |
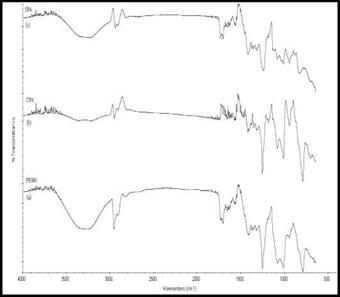
The FTIR spectra in Fig. 8 shows the spectra associated with PRM4, CP4 and SP4.The intensity of the O-H stretching vibration peak (v = 3200-3400 cm-1) decreased and the peak frequency shifted from 3292 in case of pure PVA to 3278 cm-1, 3245 cm-1 and 3230 cm-1 for PRM4, CP4 and SP4 respectively i. e. to a lower frequency as compared to that of pure PVA. This is again due to the intermolecular hydrogen bond formation, thereby decreasing the OH stretching frequency with the increase in the percent loading of the modified filler. The other characteristic peaks are given in table 6.
|
Fig. 8. FTIR Spectra of (a) PRM4 (b) CP4 (c) SP4 |
|
Groups |
PRM4 (cm-1) |
SP4 |
CP4 |
|
OH |
3278 |
3245 |
3230 |
|
C-H |
2961 |
2943 |
2959 |
|
C=O |
1711 |
1737 |
1754 |
|
O-H (bending) |
1020 |
1021 |
1020 |
|
CH2 (bending) |
1414 |
1424 |
1424 |
|
Table 6. Assignment of FTIR spectral bands for PVA/modified red mud nanocomposite membranes with different loading percentage of filler |