1.1 Preparation of boric/phosphomolybdic acid modified-red mud
A known amount of raw red mud was first washed with distilled water two to three times and then dried in an oven. When the red mud dried completely; it was treated with a 5 M solution of boric acid/ phosphomolybdic acid for 24 hours. Then it was being filtered, dried, weighed and grinded in order to get its powdered form. The weight of the red mud increased by 24 %. The powder was then sieved through 53-micron mesh to remove the larger particles. The powder so obtained was fine and acidically modified. Thus, Layered silicates and double layered hydroxides, Al2 (OH) 7 (AH) and MgAl (OH) 5 (MAH), of red mud were modified with inorganic acids. The inorganic acid modifier compatibilizes the silicate and hydroxide surface to polymer matrices and spaces the crystalline layers apart to minimize the energy needed for exfoliation process.
1.2 Preparation of organically modified-red mud
The organic modification of red mud was done by the following two steps:
i. Freshly prepared Aniline Hydrochloride is mixed with red mud with a magnetic stirrer.
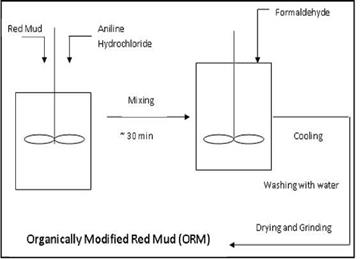
ii. Formaldehyde is then added drop by drop to the mixture with intense stirring action. The addition of aniline hydrochloride to red mud replaces the cation present in the octahedral sites of the silicate with aniline occupying the same. The formaldehyde added would form a condensation oligomer as the product with the pendent group as formaldehyde which is compatible with the polymer. The ratio of Aniline to formaldehyde is kept 1:1 so as to stop any further condensation of the aniline and formaldehyde as this could lead to the polymer blend type nanocomposite, which could have the problems associated with miscibility of polymer blends and this filler would not be universal filler for the polymer matrix. The figure 4 depicts the experimental setup for the organic modification of red mud.
|
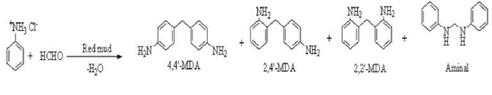
Fig. 4. Experimental setup of organic modification of red mud 2.3 Mechanism: Figure 5 showed the mechanism and condensation reaction between substituted aniline and formaldehyde. After substitution of the metal cation, condensation reaction occurs between the substituted amine group with extra hydrogen and the formaldehyde molecule to form water as a byproduct. Thus the organic entity enters the space between the silicate layers thus providing a suitable site for binding the polymer. When this filler is mixed with the polymer, the polymer chains are attracted due to the presence of the organic species at the interlayer spaces, and thus get intercalated in between the layers, which have about nanometer size openings. The reaction mechanism can be best depicted by the following sequence Aniline Hydrochloride |
_i_______________________________
M NH;+
———————– ————— ► ——————————
M NHif
Wliere,
=Silicatelay«r M =Al, Fe, Ti
|
Condensation reaction between substituted aniline and formaldehyde
Fig. 5. Mechnaism and Condensation reaction showing Organic Modification of Red Mud |