A. H. Bhat1, H. P.S. Abdul Khalil and A. K. Banthia2
1School of Industrial Technology, Universiti Sains Malaysia, Penang-11800, 2Materials Science Centre, Indian Institute of Technology Kharagpur-721302,
1Malaysia
2India
1. Introduction
Soaring prices are a reminder of the essential role that affordable products play in sustainable economic growth and higher human development. Utilization of waste materials has become more pressing than ever. Red mud is accumulating at a rate of 30 million ton annually throughout the world. Under normal conditions when 1 ton of alumina is produced from bauxite, an equal amount of red mud is generated as a waste. A further aspect is their reuse as starting materials for other products (Grjotheim & Welch, 1998; Kasliwal, & Sai, 1999). Red mud has been suggested as filler for polymer reinforcement or as a cheap adsorbent for removal of toxic metals or an acid by several researchers. Chand and Hashmi, 1999, tried to improve the mechanical properties and abrasive wear properties of polymer blend filled with red mud. Pradhan et al. 1999 had reported that activated red mud as a good adsorbent was used for adsorption of phosphate or chromium. In addition, the mechanical and thermal properties of polymers are generally improved by the addition of inorganic fillers. The challenges in this area of high-performance organic-inorganic hybrid materials are to obtain significant improvements in the interfacial adhesion between the polymer matrix and the reinforcing material since the organic matrix is relatively incompatible with the inorganic phase. Generally, a better interfacial bonding will impart better properties to a polymer composite such as high modulus, strength, and stiffness (Agag et al., 2001; Jang, 1992).This reinforcement of polymer filled with inorganic material is largely dependent on the physical interfacial phenomenon between the filler and matrix. This can be determining the degree of adhesion by physical interaction, such as active functional groups, hydrogen bonding, Lewis acid-base interactions, surface energy, and crystallite faces of filler surface at the interface (Park & Kim, 2000; Park & Cho, 2000). To increase physical interaction, various surface treatment techniques are applied, such as oxidation in acid solutions, (Donnet & Bansal, 1990) dry oxidation in oxygen, (Yuan et al., 1991) anodic oxidation, (Ishikawa & Matsumoto, 2001; Park, S. J.; Kim) and plasma treatments (Dilsiz et al., 1995). Surface modification leads to development of surface functional activity on a filler surface, resulting in modification to achieve good interfacial adhesion between the reinforcement and the matrix.
In materials research, the development of polymer nanocomposites is rapidly emerging as a multidisciplinary research activity whose results could broaden the applications of
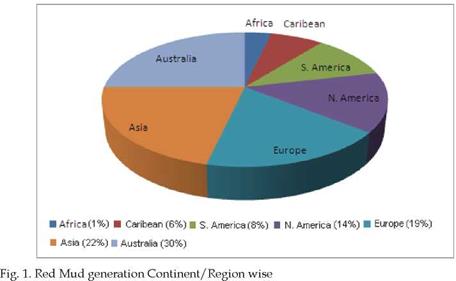
polymers to the great benefit of many different industries. The details about worldwide generation of Red Mud, country wise and region wise is shown in fig. 1.
|
|
The use of layered inorganic fillers has been a common practice in the plastics industry to improve the properties of thermoplastics. The effects of filler on the material properties of composite materials depend strongly on its particle size, shape, aggregate size, surface characteristics, and degree of dispersion. Polymer composites prepared in combination with an organic component as a matrix and an inorganic component as filler on the nanoscale (Huang et al., 1987; Suriyet et al., 1992; Novak & Ellsworth, 1993; Hajji et al., 1999) have additional advantages, such as the possibility of obtaining a material that has the advantages of both organic materials (e. g., light weight, flexibility, good moldability) and inorganic materials (e. g., high strength, heat stability, and chemical resistance)(Shao et al, 2003).
Low volume additions (<5%) of nanoparticles of layered red mud provide property enhancements with respect to the neat resin that are comparable to those achieved by conventional loadings (15-40%) of traditional fillers. Red mud (nanofiller) is a major waste material obtained during the production of alumina from bauxite by the Bayer’s process. It comprises of silicates and oxides of iron, aluminum, sodium, calcium and titanium, along with some other minor constituents. Based on economics as well as environmental related issues, enormous efforts have been directed worldwide towards red mud management issues i. e. of utilization, storage and disposal. Different avenues of red mud utilization are more or less known but none of them have so far proved to be economically viable or commercially feasible.
Thermoplastics have a big potential for applications in the industry as well as in construction, electrical applications and food packagings. One of the few disadvantages associated with the use of nanofillers, is their high cost. The present research work has been undertaken with an objective to explore the use of red mud as a reinforcing material as a low cost option. This is due to the fact that red mud alone contains all these reinforcement elements and is plentifully available.
In mechanical reinforcement major issues are the homogeneous dispersion of nanofillers in the polymeric matrix and the developments of chemical bonding or strong interaction at the nanofiller-matrix interface. In this study we have used acid modified and organically modified red mud for better homogeneous dispersion as well as enhanced material properties. The focus of this research was to experimentally characterize the two polymer nanocomposite systems and investigate the role of modification of filler in their behavior. Modified Red mud nanoparticles were dispersed in poly (vinyl alcohol) (PVA) and poly hydroxy ether of bisphenol-A (Ph) matrices.
The conventional solvent casting technique was employed to generate polymer nanocomposites. Red mud was treated with boric acid and phosphomolybdic acid to develop the acidic functional groups or active oxygen, resulting in the better dispersion of the red mud into the polymer matrices. Red mud was also organically modified with the oligomers of aniline formaldehyde, for better interaction between the filler and the polymer matrices. The particle size of the modified red mud was determined by field emission scanning electron microscopy (FESEM). The as-synthesized composite films were typically characterized by FTIR spectroscopy and X-Ray Diffraction. The morphological image of the composite materials was studied by scanning electron microscopy (SEM) and the dispersion of the modified fillers within the matrix was studied by transmission electron microscopy (TEM).The thermal properties measured by thermogravimetric analysis (TGA) showed enhanced thermal stability of a series of composite materials. The differential scanning calorimetry (DSC) showed increase in glass transition temperature and crystallization of the composite films.
The physical topography of the composite materials was studied by Atomic Force Microscopy (AFM).
Polyvinyl alcohol (PVA) is cornmercially available in dry granular or powdered form. It is a water-soluble and fully biodegradable polymer (Chen et al., 2000; Bachtsi & Kiparissides, 1996 ). PVA is having planar zigzag structure like polyethylene (Horii et al., 1992). All PVA grades are readily soluble in water. As a hydrophilic polymer, PVA exhibits excellent water retention properties. Conditions for dissolution are governed primarily by degree of hydrolysis, but they are influenced by other factors such as molecular weight, particle size distribution and particle crystallinity [Peppas & Merrill 1977]. Optimum solubility occurs at 87-89% hydrolysis. The partially hydrolyzed grades in this range exhibit a high degree of cold-water solubility. For total dissolution, however, they require water temperatures of about 185°F (85°C) with a hold time of 30 minutes. It is, in fact, a refinement of PVAcetate since the most common manufacturing process is to replace by hydrolysis (or alcoholysis) the acetate groups with hydroxyl groups. This is commonly achieved using the presence of catalytic quantities of alkali such as sodium hydroxide (which, since it acts only as a catalyst, should not in theory remain in the final product). The extent of hydrolysis will determine the amount of residual acetyl groups and this in turn apparently affect the viscosity characteristics^ Ray & Bousmina,2005] PVA exists only as a polymer; a monomer has not yet been isolated, so the chemical structure is described in Fig.2.
і
H H
I 1 1
— c —————- c—-
і і
ОН К
Fig. 2. Chemical formula of PVA
The PVA concentration in an aqueous solution is determined by the type of application. However, at concentrations greater than 10 wt%, the viscosity of the aqueous solution at room temperature is such that pouring becomes difficult. In addition to its solubility, PVA is also appreciated for its good mechanical properties in the dry state, resistance to common solvents, barrier effect in dry atmospheres, possibility of food contact for suitable grades, biodegradability. Some of the physical properties of PVA are as presented in table 1.
|
Properties |
Values |
|
Appearance |
White to cream granular powder |
|
Density |
1.23-1.30 g/cm3 |
|
Thermal stability |
Gradual discoloration above 100 oC; darkens rapidly above 150 oC; rapid decomposition above 200 oC. |
|
Coefficients of thermal expansion |
7-8 x 10-5/ oC |
|
Thermal conductivity |
0.2 W/m. k |
|
Yield Stress |
40-50 MPa |
|
Elongation at break |
100-200% |
|
Melting point |
230 oC for fully hydrolysed grades 180-190 for partially hydrolysed grades. |
|
Glass transition temperature |
75-85 oC |
|
Table 1. Physical properties of PVA |
PVA can be plasticized and processed by casting, dipping, injection and extrusion. (Biron, 2007).
The main engineering applications, possibly in combination with other polymers, are:
• Films for packing chemicals, fertilizers, herbicides, disinfectants, dyes, colorants, scalers, cosmetics etc.
• Release films for composite moulding.
• Solvent resistant tubes and pipes.
• Membranes for pumps carrying petroleum or chemical products Trade names: Elvanol, Polyviol, Mowiol, Rhodoviol.
The commercial name of poly (hydroxy ether) of bisphenol A is Phenoxy, and as a thermoplastic polymer it possesses many excellent properties such as (Zhang et al., 2002)
• Good chemical stability
• Excellent matrix material for producing polymer nanocomposites
• Thermal stability
• Tractability
• Transparency
The poly (hydroxy ether of bisphenol A) (phenoxy (Ph)) has been revealed as a polymeric matrix able to intercalate in, and partially exfoliate a commercial organically modified montmorillonite. Dispersion was attributed to chemical interactions between the Ph and the inorganic clay (Fornes et al., 2004).
Poly (hydroxy ether of bisphenol A) (Ph) based polymer nanocomposites (PN) reinforced with a layered red mud with acidic and organic modifications were prepared by conventional solvent casting technique. The best dispersion occurred in the PN where the interactions between the functional groups of the polymer matrix and those of the organic substitution of the red mud appeared to be the highest. The modulus increase is an indirect but quantitative measurement of the attained dispersion level.
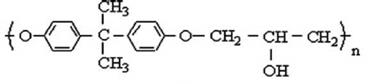
The structure of Poly (hydroxy ether) of bisphenol A is shown in fig.3.
|
Fig. 3. Poly (hydroxy ether) of bisphenol A |